|
Model Organisms Core |
 |
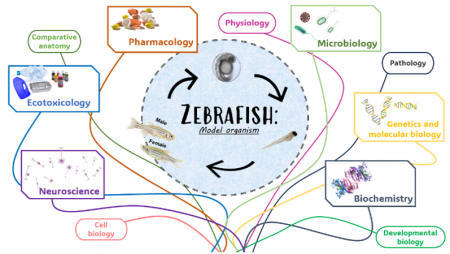
The Model Organisms Core at the Department of Biology provides the internal and outside researchers, including industry scientists, with a central resource of bacterial, plant, invertebrate and vertebrate model organisms for perusing a wide range of studies from genetics and biomedical science to ecology. The model organisms offer practical advantages such as ease of availability and breeding, ease of maintenance in the laboratory, short generation time and rapid development as well as their ability to respond well to experimental techniques and manipulations. By combining state-of-the-art genetic, physiology and ecology technologies, the Model Organisms Core offers multidisciplinary approaches to tackle challenging scientific questions effectively and to fully grasp the complexity of biological principles across the breadth of biodiversity.